caustic soda flake , Sodium hydroxide (NaOH) an inorganic compound with following synonyms
- Caustic soda
- Lye
- Sodium hydrate
caustic soda flake widely used inorganic industrial chemical. Sodium hydroxide is having certain properties like
Sodium hydroxide’s chemical formula is NaOH
NaOH molecular weight of 39.997 g/mol
Sodium hydroxide is contain ph of ~12-14
Sodium hydroxide characteristics can be mentioned as
- Raw material for various industrial products
- Co-product in chlorine synthesis
- Strong base
- Highly corrosive
- Auxiliary chemical
- Odorless material
Caustic soda is available in two forms – Caustic soda lye and Caustic soda solid. Solid form of caustic soda can be in the form ofCaustic soda flakesorCaustic soda granulesPure form of sodium hydroxide also available as
- Sodium hydroxide pellets
- Sodium hydroxide flakes
- Sodium hydroxide granule
- Sodium hydroxide solution
Caustic soda has wide variety of industrial sectors like In pulp and paper processing industry caustic soda used at the stages like bleaching process, in de-inking of waste paper, and in water treatment.
Next major industry like textile industry caustic soda is used to process cotton and synthetic fibers Caustic soda is utilized more in the soap and detergent industry.
Other caustic soda uses include
- In oil and gas industry – to remove pungent smells
- In household cleaning products
- In beverage bottles
- In home soap making
Worldwide there is high demand for caustic soda and increase in caustic soda prices, applications in daily lives this article gives the different modern caustic soda manufacturing processes.
Sodium Hydroxide solutions are produced by three different technologies
- Mercury cells
- Membrane cells
- Diaphragm cells
Each of above processes utilizes sodium chloride (NaCl) salt as the primary raw material. Electrolytic splitting of salt results in products like chlorine and sodium ion (Na+). In turn Na+ will react with water in the mercury cell to form Sodium hydroxide and Hydrogen as by product.
Mercury Cell
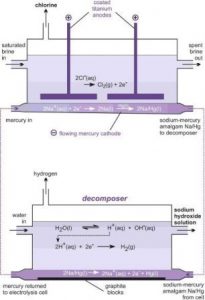
Na + e = Na
Na + Hg = Na/Hg
Soda Cell
2 Na/Hg + 2 H2O = 2NaOH + H2 + 2Hg
Diaphragm Cell
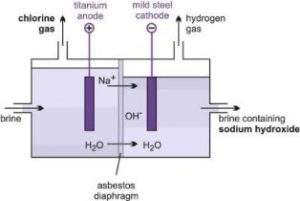
Fig. Diaphragm cell
This method produces 71 per cent of Sodium hydroxide. Diaphragm Cell process utilizes asbestos or alternate substitutes to asbestos, to separate the co-products Sodium Hydroxide (Caustic Soda) and Chlorine. The production of 50 per cent NaOH occurs primarily outside of the electrolytic cell.
The diaphragm cell produces a very weak ‘cell liquor,’ that contains 12-14 per cent, by weight, NaOH and the constant volume of NaCl salt. The cell liquor is subsequently evaporated in a three or four ‘effect’ evaporation method to a final nominal concentration of 50 per cent NaOH by weight. The surplus salt is precipitated and filtered through the evaporation method for subsequent reuse/recycle. This method produces the lowest quality electrochemical NaOH solutions.
The quality considerations with respect to the diaphragm cell produced Caustic solutions include comparatively high salt, chlorates, carbonates, and sulfates. Salt, as NaCl, concentrations are typically 1.0 per cent, with maximums ranging from 1.1 to 1.3 weight per cent, counting on producer.
The diaphragm cell created Caustic Soda , Sodium hydroxide (NaOH) is usually referred as Diaphragm Cell Grade. It is conjointly known as Commercial Grade, Technical Grade, and occasionally Technical Diaphragm or other similar combinations.
An additional ‘grade’ of Caustic Soda,Sodium hydroxide (NaOH) produced by the diaphragm cell method is the sublimate grade. The production of sublimate Grade involves the further evaporation of the 50 per cent Diaphragm Grade Caustic Soda solution to cut back the salt concentration. The higher concentration solution is then re-diluted to the 50% concentration that is commercially available as sublimate grade Caustic Soda.
Common uses include process and sewer water neutralization, textiles production, soaps and detergents and aluminum production. These uses and applications typically can confer with the Caustic Soda as any of the varied grades.
Membrane Cell
Fig. Membrane cell
This method produces approximately 13 per cent of Sodium Hydroxide. The membrane cell method utilizes a selective membrane that separates the Chlorine and Sodium ions. The membrane permits the Sodium ion to migrate across the membrane whereas keeping the Chlorine gas and salt (brine) solution in a compartment on the opposite facet of the membrane.
The Sodium ion is reacted with refined water as within the mercury cell to provide the Caustic Soda , Sodium hydroxide (NaOH). Evaporation is employed, as within the diaphragm method, to lift the concentration up to the nominal 50 weight per cent solution. The salt concentrations are not targeted as considerably during this evaporation method attributable to the selective diffusion nature of the membranes as well as the reduced quantity of evaporation needed during this method opposed to the diaphragm evaporation.
The Caustic Soda produced by the membrane cell process is most typically brought up as Membrane Grade. It conjointly contains a growing acceptance as a Rayon Grade product in all areas outside of rayon fiber production.




